Authors: Joseph W. Metro Robert J. Hill

Introduction
For many years, Democratic Party lawmakers have sought to limit prescription drug prices through bills which would require the federal government’s “negotiation” of such prices with pharmaceutical manufacturers. The IRA contains provisions to implement that goal, albeit only with respect to prices paid under Medicare Parts B and D, and only for certain narrowly defined high-expenditure drugs, with prices negotiated for a limited number of such drugs each year. Moreover, the process appears to be less of a negotiation than a mechanism through which the government can specify the maximum prices manufacturers will receive for drugs paid for by Medicare, with manufacturers potentially facing the limited alternatives of agreeing to the price the government demands, paying punitive excise taxes, or withdrawing their products from coverage under the Medicare and Medicaid programs.
The IRA establishes a process by which the Secretary of Health and Human Services (Secretary) will identify certain single source drugs accounting for significant Part B and/or Part D expenditures, and will negotiate a “maximum fair price” (MFP) for a specified number of such drugs with their respective manufacturers.3 The MFPs for the initial set of such “selected drugs” will be negotiated over a ten month period beginning October 1, 2023 and applicable in 2026. Once negotiated, MFPs will continue in effect until at least nine months after a selected drug is subject to generic or biosimilar competition. The MFPs cannot exceed certain statutory ceilings, but generally are not subject to any floors. Manufacturers will be required to make the MFPs available to pharmacies and providers for dispensing or administration to eligible patients under Parts B and D, although the operational details relating to how that will occur are yet to be determined – with significant potential implications for pharmacies, wholesalers and other drug supply chain participants.
Negotiation Process Timeline
Under the Program, the Secretary will establish an annual price negotiation process with respect to a specified number of “selected drugs.” The MFPs negotiated for those selected drugs first become applicable during the “initial price applicability year” (referred to herein as “IPAY”) for such drugs. The first IPAY is calendar year 2026. The IPAY and each subsequent year during which a drug is a selected drug is the “price applicability period” for such selected drug.
The IRA specifies a somewhat different negotiation timeline for the 2026 IPAY than for 2027 and each subsequent IPAY. The two timelines are illustrated in Figures 1 and 2, and described in detail below.
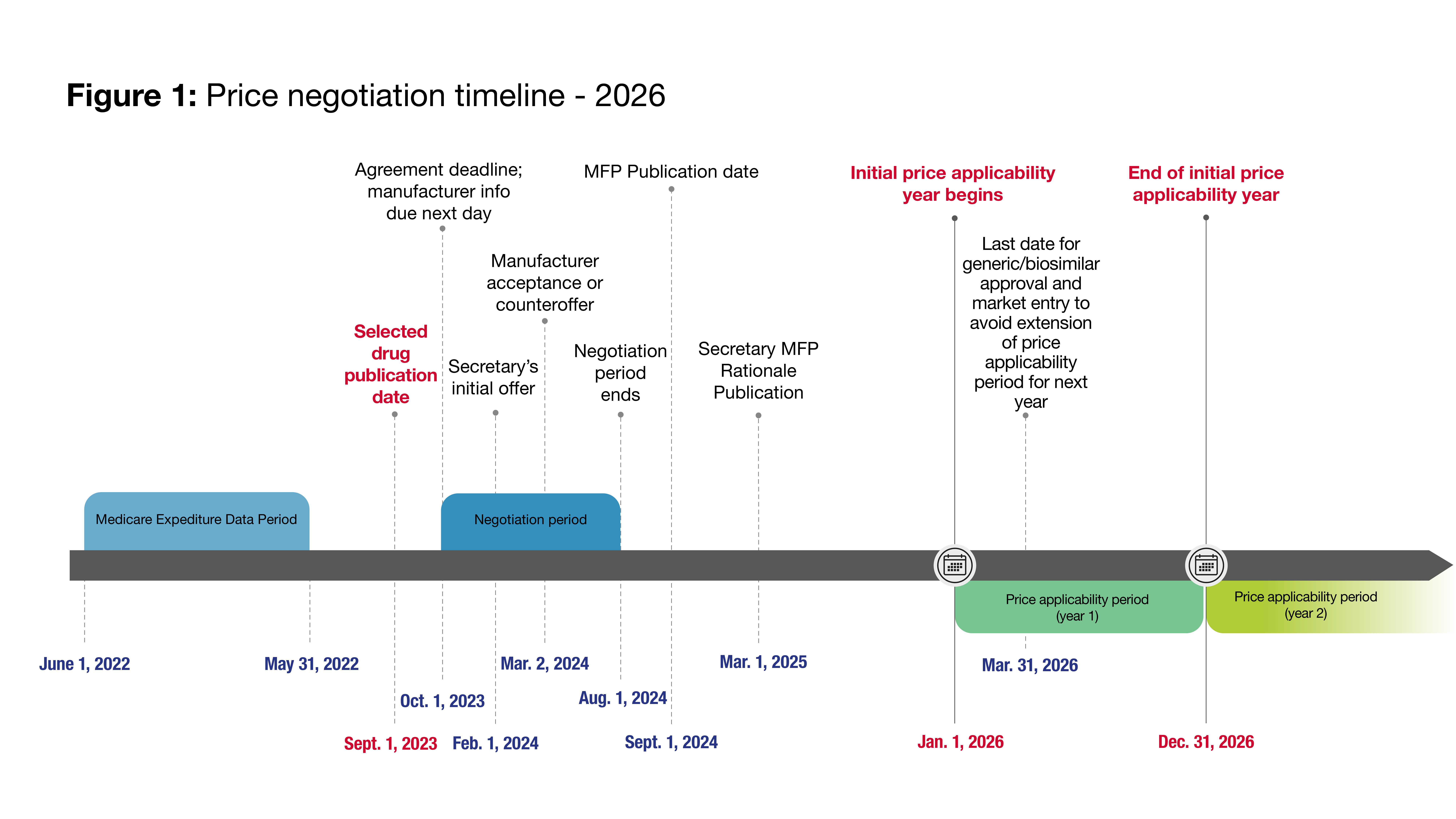
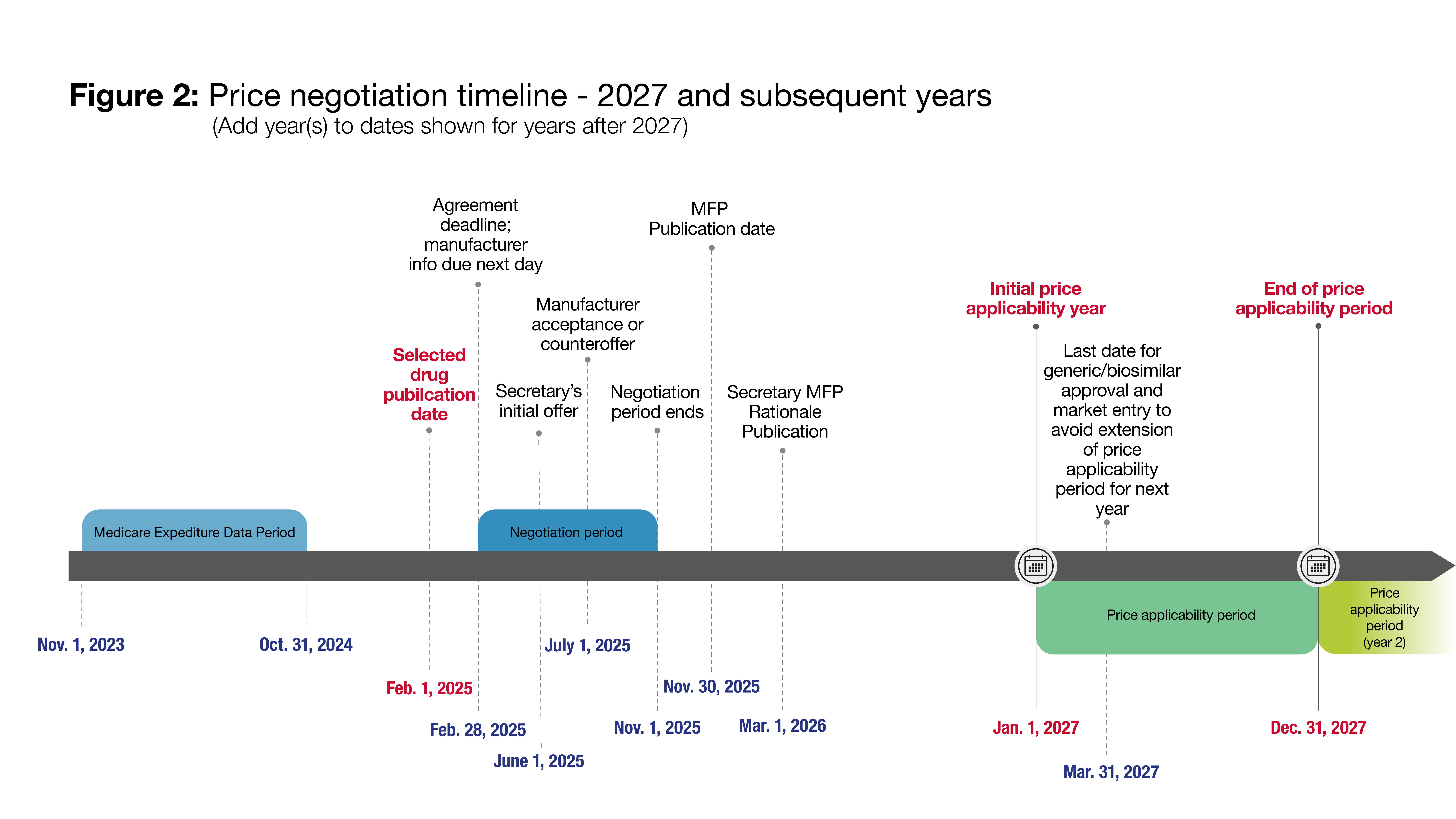
The negotiation process will begin with the “selected drug publication date,” which is September 1, 2023 for IPAY 2026, and for each subsequent IPAY, February 1 of the calendar year that begins two years prior to the IPAY (e.g., for IPAY 2027, February 1, 2025). On that date, the Secretary will publish a specified number of “selected drugs” consisting of those “negotiation-eligible drugs” (defined in the next section) which had the highest level of expenditures under Medicare Part D or Medicare Parts B and D combined, as follows:
Number of Selected Drugs for each Initial Price Applicability Year:
- 2026: 10 (based on highest Part D expenditures)
- 2027: 15 (based on highest Part D expenditures)
- 2028: 15 (based on highest combined expenditures under Parts B and D)
- 2029/later: 20 (based on highest combined expenditures under Parts B and D)
The foregoing determinations for each year exclude drugs that are already selected drugs. Additionally, if there are less than the specified number of negotiation-eligible drugs for the given year, then the number of selected drugs for that year will be the total number of negotiation-eligible drugs. Expenditures will be determined by the Secretary based upon data for the period of June 1, 2022 through May 31, 2023 for IPAY 2026, and for 2027 and each subsequent IPAY, based upon data for the most recent 12-month period ending no later than the previous October 1 (e.g. for IPAY 2027, for the period of November 1, 2023 through October 31, 2024 or an earlier 12-month period, subject to certain exceptions and specifications discussed in the next section).
Within approximately a month after the Secretary publishes the list of selected drugs, the IRA provides that “the Secretary shall enter into agreements” with the manufacturers of such selected drugs; as noted below, manufacturers who fail to do so are subject to excise taxes. Specifically, for IPAY 2026, such agreements must be entered into by October 1, 2023, and for 2027 and each subsequent IPAY, such agreements must be entered into by February 28 following the selected drug publication date. The “negotiation period” for the selected drug begins on the earlier of the specified deadline for entering into the agreement or the date the agreement is actually entered into, and ends on August 1, 2024 for IPAY 2026 or, for 2027 and each subsequent IPAY, by November 1 of the year that begins two years prior to the initial price applicability year (e.g., for IPAY 2027, November 1, 2025).
The negotiation process itself is subject to statutory timelines.
First, by October 2, 2023 for IPAY 2026, and for subsequent IPAYs by March 1 after the selected drug publication date (e.g., March 1, 2025 for 2027), the manufacturer must provide information to the Secretary concerning the selected drug, consisting of information on the Veteran’s Health Administration (VHA) non-federal average manufacturer price (NFAMP) for the drug4 and other “information that the Secretary requires to carry out the negotiation … process.”5
Second, by February 1, 2024 for IPAY 2026, and for subsequent IPAYs by June 1 following the selected drug publication date (e.g., June 1, 2025 for 2027), the Secretary must provide a written initial proposal for the MFP and “concise justification” for such proposed MFP based on the factors the Secretary is required to consider (described below).
Third, the manufacturer must accept the initial offer or provide a written counteroffer within 30 days of its receipt, along with its own justification based upon the specified factors. The Secretary must respond in writing to such counteroffer. This process may continue during the negotiation period.
Finally, within a month after the end of the negotiation period (by September 1, 2024 for IPAY 2026, and November 30 for subsequent IPAYs), the Secretary must publish the negotiated MFP. By March 1 of the year prior to the initial price applicability year (e.g., March 1, 2025 for IPAY 2026, and March 1, 2026 for IPAY 2027), the Secretary must publish the rationale for the MFP, based upon the statutory factors to be considered in the negotiation.
The MFP continues in effect (subject to annual inflation adjustments and renegotiation) until a drug ceases to qualify as a “selected drug.” A product generally continues to be a “selected drug” until the first year that begins at least nine months after the Secretary determines that a generic drug or biosimilar has been approved and marketed, for which the selected drug is the listed or reference product.
“Selected Drugs” Subject To Negotiation
In general
While the Secretary must annually negotiate MFPs with respect to a specified number of “selected drugs,” the process of “selection” actually begins with the Secretary’s identification and ranking of “negotiation-eligible drugs.” Negotiation-eligible drugs are in turn defined based on “qualifying single source drugs.”
Subject to the exclusions noted below, “qualifying single source drugs” include both:
(i) Drugs marketed under a new drug application (NDA) approved at least seven years prior to the selected drug publication date, which are not otherwise the “listed” reference drug for a generic equivalent approved and marketed under an abbreviated new drug application (ANDA); and
(ii) Biologics marketed under a biologics license application (BLA) under Section 351(a) of the Public Health Service Act (“PHS Act”) approved at least eleven years prior to the selected drug publication date which are not the reference product with respect to any approved and marketed biosimilar.6
The statute provides that an “authorized generic drug” will be treated the same as its respective qualifying single source drug, and includes a statutory definition of “authorized generic drug” which also encompasses biologics.7
The definition of qualifying single source drug excludes:
(a) Orphan drugs for which the only approved indication is the rare disease or condition that resulted in orphan status;
(b) “Low spend Medicare drugs,” defined as drugs or biologics for which the total expenditures under Parts B and D are, for IPAY 2026, less than $200 million for the period June 1, 2022 through May 31, 2023, and for subsequent IPAYs, such $200 million amount as adjusted for CPI-U inflation;8 and
(c) Biologics derived from human whole blood or plasma.
“Negotiation-eligible drugs” are generally defined as those qualifying single source drugs which are among the 50 such drugs having the highest total expenditures under Part D or Part B, although for IPAYs 2026 and 2027, such term includes only the top 50 expenditure qualifying single source drugs under Medicare Part D. Part D expenditures are defined to include “gross covered prescription drug costs” as defined under Part D statutory provisions, referring to amounts paid by or on behalf of the plan or beneficiary,9 and Part B expenditures exclude payments for products that are bundled into the payment for another service or item. Expenditures are measured including all dosage forms and strengths of the drug, including new formulations such as extended release formulations.10
However, for IPAYs 2026-28, negotiation-eligible drugs do not include any “small biotech drug,” described as a product for which 2021 expenditures under Part B or D (as applicable) both (i) represent less than one percent of total Part B or D expenditures (respectively) for all drugs and (ii) account for at least 80 percent of the expenditures for all of the manufacturer’s applicable drugs covered under its coverage gap discount agreement (in the case of Part D drugs) or at least 80 percent of the total expenditures under Part B for all qualifying single source drugs of the manufacturer (in the case of Part B drugs). All entities treated as a single employer for tax purposes will be considered a single manufacturer for purposes of this exception, and any exempt manufacturer acquired after 2021 by a manufacturer that is not a “specified manufacturer”11 under Part D will lose its exemption effective January 1, 2025 (or the year following the acquisition).
To determine the selected drugs for an IPAY, subject to the exceptions described below, the Secretary must rank the negotiation-eligible drugs based on their amount of Part D (for IPAYs 2026 and 2027) or combined Part B and Part D (for 2028 and subsequent IPAYs) expenditures, and then “select” the specified number of products “with the highest such rankings.” However, if the Secretary determines, before the end of the negotiation period, that a generic equivalent or biosimilar for a selected drug included on the list published by the Secretary has been approved and is marketed, then that drug will not be subject to negotiation of an MFP. Notably, the statute appears to provide that a selected drug for which generic or biosimilar entry occurs after the negotiation period but before the beginning of the applicable IPAY will still be treated as a selected drug, subject to the applicable MFP. 12
Special rules relating to deferral of biological product selection for biosimilar competition
The statute also includes a process by which the “selection” of certain biologics may be deferred for up to two years, if the Secretary determines that there is a “high likelihood” (as defined) that a biosimilar competitor will be approved and marketed within two years of the selected drug publication date. The deferral process must be initiated by a request from the biosimilar manufacturer which includes information enabling the Secretary to consider the manufacturing schedule and marketing position of the biosimilar.
The deferral process is only available with respect to “extended monopoly” biologics, which are products (other than vaccines and products subject to a selected drug agreement with the Secretary before 2030) that were first licensed at least 12 but no more than 16 years prior to the IPAY. A “high likelihood” is defined to mean that a biologics license application has been accepted or approved for the biosimilar, and that there is clear and convincing evidence that the biosimilar will be marketed in the statutory time frames. If the Secretary determines that there is a “high likelihood” of such biosimilar entry within such two year period, the Secretary must delay the inclusion of the reference biologic as a selected drug for one year. However, no deferrals (or extensions of deferrals) may be issued where the biosimilar and reference biological manufacturer are the same entity, where the two manufacturers have entered into an agreement which requires or incents the biosimilar manufacturer to submit a deferral request or limits biosimilar sales quantities, or if the biosimilar is not marketed within one year of its licensure.
If the biosimilar has not been licensed and marketed during the initial one-year deferral period, the biosimilar manufacturer may request that the Secretary issue a second one-year deferral. In such case, the Secretary must evaluate whether there is still a high likelihood of licensing and marketing, and also whether the biosimilar manufacturer has made significant progress toward licensure and marketing (again, on the basis of clear and convincing evidence). If so, the Secretary may defer the selection of the reference biological for an additional year (unless the reference biological transitioned to “long monopoly” status (i.e., 16 or more years have elapsed between its approval and the IPAY) during the delay period).
If the biosmilar is not approved and marketed during the first year delay and the Secretary determines that a high likelihood of timely approval and marketing no longer exists (or lack of significant progress has been made), or if the second year delay is granted and the biosimilar is not approved and marketed by the selected drug publication date two years after the initial deferral, the reference biological manufacturer entering into a selected drug agreement with thSecretary must pay a rebate for the IPAY(s) to which the deferral applied.13
The Maximum Fair Price
Once a manufacturer’s product has been “selected” and the manufacturer has entered into an agreement with the Secretary, the Secretary and the manufacturer must negotiate an MFP for that product and provide access to that price to Medicare Part D beneficiaries and to pharmacies and providers that dispense, administer or furnish the product to Part D or Part B beneficiaries.
Methodology and Process. The IRA requires the Secretary to develop and use a “consistent methodology and process” for negotiations under the Program.
Factors to be considered in negotiations. The Secretary must consider specified factors when determining MFP offers and counteroffers. These consist of:
- Data on research and development costs for the product and the extent to which the manufacturer has recouped such costs;
- Data on current production and distribution costs for the drug;
- Prior federal financial support for the discovery and development of the drug;
- Data on pending and approved patent applications and other FDA-recognized exclusivities for the drug;
- Market, revenue and sales data; and
- To the extent available with respect to the drug and its therapeutic alternatives, the degree to which the drug represents a therapeutic advance and the costs of therapeutic alternatives, FDA-approved prescribing information, comparative effectiveness data with respect to specific populations such as the elderly, disabled or terminally ill (but not in a manner that treats extending their lives as having lower value), and the extent to which the product and its therapeutic alternatives addresses unmet medical needs.
Ceilings on MFPs. Significantly, the Secretary may not offer or agree to an MFP that exceeds certain statutory ceilings. Specifically, the maximum MFP for the IPAY may not exceed the lower of:
(i)(A) In the case of a Part D drug, the enrollment weighted average negotiated prices (i.e., prices payable to dispensing pharmacies) for the selected drug, net of all price concessions (e.g., voluntary manufacturer rebates) received by the Part D plan sponsor or its pharmacy benefit manager (“PBM”), for the most recent year for which such data is available, under all Part D prescription drug or MA-PD plans; or
(B) In the case of a Part B drug, the average sales price (ASP) or wholesale acquisition cost (WAC) of the drug used to determine its payment amount under Part B for the year prior to the selected drug publication date;14 or
(ii) The “applicable percent” below of an inflation-adjusted NFAMP for the drug (calculated as described below):
(A) 75 percent, for “short-monopoly drugs” and vaccines (defined as selected drugs other than extended-monopoly drugs or long-monopoly drugs);
(B) 65 percent, for “extended monopoly drugs” (defined as selected drugs for which FDA approval occurred between 12 and 16 years ago, other than vaccines and selected drugs for which the manufacturer had a selected drug agreement with the Secretary for an IPAY before 2030); and
(C) 40 percent, for “long-monopoly drugs” (defined as selected drugs for which FDA approval occurred at least 16 years prior to the IPAY, other than vaccines).
The inflation-adjusted NFAMP against which the applicable percentage is multiplied is: (i) for IPAY 2026, the average (over four quarters) NFAMP for 2021 (or for the first full year after marketing, for products without a full-year NFAMP for 2021) adjusted by the change in the CPI-U from September 2021 (or from December of the first full year after market entry) to September 2022; or (ii) for IPAY 2027 and subsequent IPAYs, the lower of (A) the average NFAMP for 2021 (or for the first full year of marketing) adjusted by the change in the CPI-U from September 2021 (or December of the first full year of marketing) to September of the year preceding the selected drug publication date (i.e., September 2024 for IPAY 2027) or (B) the average NFAMP for the year prior to the selected drug publication date.
Notably, under current law, the VA, Department of Defense, and Public Health Service may purchase a single source drug at a price no higher than 76 percent of the prior year’s NFAMP, less an additional amount for year-over-year price increases in excess of the CPI-U. See 38 U.S.C. § 8126. Thus, the highest ceiling above (75 percent) should effectively place the Medicare program on a price footing somewhat comparable to that of these federal agencies for selected drugs.
Temporary floor for MFPs of Small Biotech Drugs. While there is no floor on the MFP that the Secretary may propose for selected drugs generally, for IPAYs 2029 and 2030, the IRA does specify a statutory floor for the MFP of “small biotech drugs” excluded from the definition of “negotiation eligible drugs” for IPAYs 2026-2028, as described above. For such products, the minimum MFP will be 66 percent of their average NFAMP for 2021, adjusted for inflation as described above.
Annual Adjustment of the MFP for Inflation. For each year during a price applicability period after the IPAY, the prior year’s MFP will carry over, subject to an inflation adjustment, unless the MFP is renegotiated for such year (as described below). The Secretary must publish the new MFP by November 30 of the year two years prior to the year for which it is applicable. The inflation-adjusted MFP will be the MFP for the previous year increased by the percentage increase in CPI-U for the 12-month period ending with the July prior to the date of such publication.15
Renegotiation of the MFP. The MFP may be subject to renegotiation. Beginning in 2028, a drug’s MFP is subject to renegotiation if (i) the drug secures a new indication, (ii) the drug’s status changes to an extended monopoly drug or to a long monopoly drug, or (iii) the Secretary determines that there has been a material change in any of the factors (described above) to be considered by the Secretary in negotiating an MFP. The Secretary must “select” for renegotiation all drugs in category (ii), but has discretion whether to renegotiate MFPs for the other categories based on whether it would result in a “significant change” in the MFP. The statute provides that the Secretary must establish a process for renegotiation comparable to that for original price negotiations.
Implementation of the MFP
Provision of MFP to providers and beneficiaries. Under the agreement with the Secretary, the manufacturer must “provide access” to the MFP to:
(i) Individuals enrolled in a Part D prescription drug plan or MA-PD plan to whom such drug is dispensed during the price applicability period “and to pharmacies, mail order services, and other dispensers, with respect to” such individuals; and
(ii) To “hospitals, physicians and other providers of services and suppliers with respect to” individuals enrolled in Part B, including individuals enrolled in a Medicare Advantage plan, who are furnished or administered such drug under Part B during the price applicability period.
However, a manufacturer is not required to “provide access” to an MFP with respect to such individuals “who are eligible to be furnished, administered, or dispensed such selected drug at a covered entity described Section 340B(a)(4) of” the PHS Act, or to such covered entity, if the 340B ceiling price for the selected drug is lower than the MFP.16
With respect to Part D enrollees, the MFP is required to be provided to beneficiaries at the point of sale, and the “negotiated price” payable to the dispensing pharmacy may not exceed the MFP plus any dispensing fees. With respect to Part B beneficiaries, the MFP is used to determine the payment amount for selected drugs that are single source drugs or biologics for which the payment amount would otherwise be 106 percent of ASP or WAC (i.e., at 106 percent of MFP).17
Significantly, the IRA is completely silent with respect to the mechanism by which manufacturers must make these prices available. With respect to Part D beneficiaries, the fact that the MFP must be made available to both the pharmacy and the enrollee, and that the negotiated price payable to the pharmacy cannot exceed the MFP, may implicitly indicate that the pharmacy cannot take any margin (over its acquisition cost) on the ingredient cost for the drug.18 However, those limitations do not appear to apply to dispensing fees, and consequently pharmacies may seek to negotiate higher dispensing fees for selected drugs, to compensate for their loss of margin on ingredient costs.
More generally, however, it is not clear how a manufacturer that sells selected drugs to distributors and providers can ensure “access” to the MFP for only Part B and D beneficiaries, without also providing that price to individuals covered under commercial health insurance (or who lack or are not using health insurance). Some possibilities in this regard may include some form of rebates, distributor chargebacks, direct distribution pricing, or “replenishment” models similar to those used in the context of the 340B drug discount programs, or a combination of such approaches. These mechanisms may require further contracting between or among manufacturers, distributors, PBMs, pharmacies, physicians, hospitals or other providers, and could also involve changes to claim adjudication or other industry standards. The IRA provides that the Secretary’s administrative duties include “establishment of procedures to carry out the provisions” of the statute relating to selected drugs with respect to Part B and D beneficiaries, suggesting CMS may take the lead in this regard. However, inasmuch as industry participants will need to actually carry out any mechanism to apply MFPs as contemplated by the statute, we expect that CMS will seek input from stakeholders as it works through these issues.
Application of MFP to different strengths and dosage forms. The IRA provides that the Secretary shall establish procedures to compute and apply the MFP across different strengths and dosage forms of a selected drug and not based upon the specific formulation or package size of the selected drug.
Inclusion of selected drugs on Part D formularies. The IRA requires that Part D plan formularies include each selected drug during a price applicability period, subject to their right to remove such drug if they place its generic equivalent on formulary (i.e., during any period after the generic is introduced to the market, but the selected drug’s price applicability period has not ended). Notably, this provision does not preclude a Part D plan sponsor from placing a selected drug on a disadvantageous cost-sharing tier (e.g., the non-preferred brand tier) or application of utilization management criteria for coverage of a selected drug to be available, such as prior authorization, step therapy, or quantity or duration limits.19
Treatment under AMP, Medicaid best price and ASP. The IRA provides that MFPs are excluded from the “average manufacturer price” (AMP) reported by manufacturers under the Medicaid rebate statute, but included in the determination of the “best price” under that statute. The IRA does not revise the statutory definition of ASP to specify how MFPs are to be treated thereunder; inasmuch as there is no clear exclusion of MFPs from the general language of that definition, it is possible that MFPs will be included in the calculation of ASP.
Excise Taxes and Civil Monetary Penalties
Although the Program appears to be technically voluntary in that a manufacturer theoretically might be able to decline to enter into a selected drug agreement with the Secretary or “refuse to agree” to an MFP for its product, the IRA imposes severe excise tax consequences were that to occur. Specifically, the IRA provides for a tax on sales of a “designated drug” by a “manufacturer, producer, or importer” during a “noncompliance period.”20 Designated drugs are negotiation-eligible drugs that are included on the Secretary’s selection list. Noncompliance periods include: (i) days during which the manufacturer has not entered into a selected drug agreement after the statutory deadline for doing so; ii) days after the end of the negotiation period (or renegotiation period) during which the manufacturer has not agreed to an MFP (or renegotiated MFP); and (iii) days during which a manufacturer’s submission of information to the Secretary for negotiation purposes is overdue.
The amount of the tax is equal to an amount such that an “applicable percentage” is equal to the ratio of (i) the tax amount, divided by (ii) the sum of the tax amount and the price for which the product was sold. The applicable percentage depends on the duration of noncompliance on the date of the sale: 65 percent if within the first 90 days; 75 percent if between days 91-180; 85 percent if between days 181 and 270; and 95 percent on any subsequent day. Based upon our interpretation of this provision, the excise tax would actually be a multiple of the sales price, ranging from approximately 186 percent to 1,900 percent of the sales price.
Excise taxes will not be deductible. However, the statute also provides that no tax will be assessed for periods for which a manufacturer has terminated its Medicare Part D coverage gap and discount agreements and its Medicaid rebate agreement (effectively precluding coverage of all of the manufacturer’s products under Medicare and Medicaid). Consequently, it appears that a manufacturer can avoid the tax only by opting out of Medicare and Medicaid entirely.
The IRA also authorizes the Secretary to impose steep civil monetary penalties (CMPs) for various types of Program noncompliance. First, although the statute does not specify a mechanism for manufacturers to make MFPs available to eligible individuals or dispensing providers, it authorizes a CMP for failing to provide access to the MFP, equal to ten times the difference between the price charged for the noncompliant units and the MFP. Second, if a manufacturer fails to comply with its obligations under its selected drug agreement with the Secretary to comply with Program administration requirements established by the Secretary (including by failing to submit as-yet undefined negotiation information to the Secretary), the statute authorizes a CMP of $1 million per day. Third, if a manufacturer knowingly provides false information with respect to the Secretary’s application of the “single employer” or acquisition rules applicable to determination of negotiation-eligible drugs and small biotech drugs, it may be subject to a CMP of $100 million. Finally, if a reference biologic’s “selection” was deferred and its manufacturer was required to pay rebates for the period of such deferral, the statute authorizes a CMP for failure to pay such rebates, equal to ten times the amount of the rebate due.
Preclusion of Judicial/Administrative Review
Finally, the IRA provides that there shall be no administrative or judicial review of the determination of product “units,” the selection of drugs for negotiation, the determination of negotiation-eligible drugs, the determination of qualifying single source drugs, the determination of the MFP, or the determination or selection of renegotiation-eligible drugs.
Potential Implications
The IRA provisions related to selected drugs and MFPs are complicated, and numerous critical details remain to be determined. However, at this stage we note the following preliminary observations.
- How low can it go? As noted, the MFP which the Secretary demands is generally not subject to any floor, and absent withdrawal from the Medicare and Medicaid programs, manufacturers have no economically viable way to refuse to agree to the MFP that the Secretary proposes. Consequently, while manufacturers often may be willing to live with the ceiling prices applicable to MFPs, the uncertainty of whether the Secretary will demand a lower MFP, and how low that price might be, creates a serious potential threat to Medicare revenue – particularly for the first manufacturers to go through the negotiation process.
- Is there duplication with “voluntary” rebates paid to the Part D plan? Manufacturers pay rebates on most qualifying single source drugs to Part D plan sponsors (directly or through their PBMs) on a voluntary basis to secure placement on plan formularies.21 Inasmuch as MFPs are subject to a ceiling that reflects those rebates for a recent period, a manufacturer of a selected drug that agrees to an MFP and nevertheless continues paying voluntary rebates at the same rate may effectively double the level of rebates it is providing. We expect many manufacturers will seek to terminate voluntary rebates on selected drugs subject to an MFP, but PBMs and Part D plan sponsors may resist such attempts, in some cases pursuant to multi-year rebate contracts negotiated prior to passage of the IRA.22 Moreover, even if a manufacturer has the ability, as a contractual matter, to cease paying voluntary rebates on a selected drug, in some cases doing so could result in disadvantageous formulary placement vis-à-vis competitive products. Consequently, manufacturers of selected drugs may find it necessary to pay at least some voluntary rebates, in addition to providing an MFP, to maintain their Part D sales levels, driving their net revenue even lower than the MFP.
- How can price negotiation be avoided? In light of the foregoing uncertainties and consequences, we expect that many manufacturers will consider ways to avoid having their products selected for negotiation, or exempted from negotiation if and when it occurs. Most significantly, a product for which a generic equivalent or biosimilar is approved and marketed will not be selected for negotiation. Consequently, some manufacturers may find it advantageous to relax patent defenses or other barriers to entry to allow entry of a generic or biosimilar. Manufacturers conceivably could even wait until they learn what MFP the Secretary proposes for their product before making a final decision on allowing a generic or biosimilar to enter the market, although that may pose some risk, since failure of a generic or biosimilar to be approved and marketed prior to the end of the negotiation period may force the manufacturer to agree to the MFP for at least one year. Other options for avoiding negotiation may also be considered.
- Are there ways to challenge the Program in court? Despite the limitation on judicial or administrative review under the statute, manufacturers or their trade associations may seek to challenge the Program or its administration in court. While it is beyond the scope of this Client Alert to evaluate the potential bases for such a challenge or their likelihood of success, there is a possibility that such an action may result in the price negotiation provisions being overturned or delayed.
- Potential 340B implications. The provisions of the law exempting manufacturers from providing an MFP when they are providing lower prices under the 340B program may implicitly require the Secretary to establish a mechanism for identifying when a 340B discount is provided on a selected drug dispensed or administered to a beneficiary – something that Part D plans and their PBMs generally cannot determine currently.23 Such a development could have collateral effects on manufacturer payment of rebates (which frequently exclude scripts filled with 340B-discounted product), pharmacy reimbursement (which PBMs may seek to reduce when 340B-discounted product is used) and other issues.
- Limited timeframe for complicated Program implementation. As noted, the basic mechanism for MFPs to be made available in accordance with the statute remains to be determined, along with establishment of the necessary contracts and administrative mechanisms. While MFPs will not become applicable prior to 2026, the first set of selected drugs will be announced and negotiation will begin in only a year’s time, during which the Secretary will need to make numerous decisions. These may have a variety of direct and indirect implications for various industry participants.
- Pub. L. No. 117-169.
- Client alerts Part 1, Part 2 and Part 3 are available on the reedsmith.com website.
- The Secretary has generally delegated functions relating to the administration of Medicare and Medicaid to the Centers for Medicare and Medicaid Services (CMS). As such, we expect that the price negotiation process will similarly be administered by CMS, in practice.
- As noted further below, the IRA provides that the MFP for a selected drug may not be more than the specified ceiling amount, calculated as a percentage of the drug’s NFAMP. The IRA references 38 CFR § 8126(h)(5) for the definition of NFAMP, which defines such term as the weighted average price, during a given period, of a single form and dosage unit of a drug that is paid by wholesalers in the United States to the manufacturer, taking into account any cash discounts or similar price reductions, but not taking into account any prices paid by the federal government or any prices found by the Secretary to be merely nominal in amount.
- This open-ended language may effectively be limited by provisions setting forth the factors the Secretary shall consider in negotiating the MFP, as described below.
- Note that a biosimilar is outside of the definition, and consequently biosimilars cannot be selected drugs.
- The statutory definition is not a model of clarity. Among other things, the biologics included in the definition are limited to those that are “marketed, sold or distributed directly or indirectly to retail class of trade”; query whether biologics that are sold only to hospitals, physicians and other providers for administration to patients are outside of that definition. See Section 1192(e)(2)(B)(ii)(II) of the Social Security Act (SSA), as added by the IRA.
- “CPI-U” as used herein refers to the consumer price index for all urban consumers (all items, United States city average), as published by the Bureau of Labor Statistics.
- It is not clear to us whether the Secretary will determine Part D expenditures net of manufacturer rebates, or prior to application of manufacturer rebates. While the Part D statutory definition referenced appears not to refer to the “net” amount, we note that the CMS regulatory definition of that term at 42 CFR § 423.308 does relate to such net cost, inasmuch as it requires that the costs be “actually paid,” a term defined in the same section to mean the costs are net of rebates and other discounts.
- As noted above, negotiation-eligible drugs do not include drugs that are already selected drugs.
- Under the Medicare benefit design reforms contained in the IRA, a “specified manufacturer” is generally defined to be a manufacturer whose products accounted for less than one percent of Medicare Part B and Part D drug spending.
- The statute provides that a selected drug for which generic or biosimilar entry occurs before or during the negotiation period “shall continue to be considered a selected drug under this part with respect to the number of negotiation-eligible drugs published on the list” by the Secretary; this effectively means that such a selected drug will not be replaced with another negotiation-eligible drug not on the list, for which an MFP would then be negotiated. Additionally, however, we note that a selected drug for which a generic or biosimilar is approved and marketed during the negotiation period may still technically fall within the definition of “selected drug” for purposes of IRA provisions not relating to the MFP, such as the reset of the benchmark quarter relating to calculation of inflation rebates.
- The amount of the per unit rebate equals (i) in the case of Part D biologics, 75 percent of the difference between the Medicaid average manufacturer price and the MFP (as adjusted) that would have applied during the deferral period(s), and (ii) in the case of Part B biologics, 80 percent of the difference between the Medicare ASP-based payment amount and the MFP (as adjusted) that would have applied during the deferral period(s). However, if the reference biologic qualifies as a “long monopoly” drug (see definition below) at the time of its ultimate inclusion as a selected drug, the amount of the rebate will be 65 percent of such biologic’s 2021 NFAMP, increased by the change in CPI-U from September 2021 to the September prior to the selected drug publication date that would have applied had there been no delay. In the case such a biologic does not have a NFAMP for 2021, the rebate would be 65 percent of its NFAMP for the first full year following market entry, increased by the change in CPI-U from December of such year to the September prior to the selected drug publication date that would have applied if there had been no delay.
- The statute technically refers to the “payment amount under section 1847A(b)(4) [of the SSA] for the drug or biological product,” but that subsection only refers to the lesser of the ASP or WAC portion of the payment amount formula, before application of the 106 percent multiplier, which appears in section 1847A(b)(1). Presumably the Secretary will determine how to interpret the provision in light of such ambiguity. However, we believe it is unlikely that this element of the ceiling will be lower than the applicable NFAMP ceiling.
- It does not appear that the MFP would be reduced if there is deflation (i.e., if the CPI-U over the 12-month period were to fall), inasmuch as the statutory language only refers to increasing the MFP to reflect an increase in CPI-U.
- Section 1192(d) of the SSA, as added by the IRA. Where the MFP is below the 340B ceiling price, the manufacturer is required to provide access to the MFP “in a nonduplicated amount to the ceiling price” (Section 1194(d)(2)). Consequently, where an MFP is below a 340B ceiling price, it appears that the IRA contemplates that the Program would be administered to still provide for a 340B discount on the selected drug unit, with an additional discount to reduce the price to the MFP, rather than by providing that no 340B discount is payable.
- For both Part B and Part D beneficiaries, the Secretary is directed to establish a mechanism to ensure that the MFP is applied before any coverage or financial assistance under other health benefit plans or programs that provide coverage or financial assistance for the purchase of drug coverage, and before any other discounts.
- If a manufacturer were to sell the selected drug to the pharmacy at a price below the MFP, the pharmacy could potentially take that additional discount as its margin, and dispense the drug at the MFP.
- We also note that the IRA amended the Part D “non-interference” clause to permit the Secretary to “institute a price structure for the reimbursement of covered part D drugs” as provided under the selected drug provisions.
- Note that this tax might not apply to bulk sales of product made by a manufacturer prior to a noncompliance period, in anticipation of noncompliance or a challenge to some aspect of the Program. However, the IRA provides that the Secretary of the Treasury may treat sales timed for the purpose of avoiding the excise tax as occurring during a noncompliance period, and therefore subject to such tax.
- In some cases, manufacturers also pay rebates to Medicare Advantage organizations on Part B drugs covered under a Medicare Advantage plan.
- Apart from MFPs, manufacturers may seek to renegotiate rebate agreements in light of the other changes made by the IRA, such as imposition of inflation rebates and replacement of coverage gap discounts with new mandatory manufacturer discounts payable during both the initial coverage and catastrophic phases (after satisfaction of the deductible) of the Part D benefit.
- Similar exemptions of 340B-discounted drugs from inflation rebates will provide a similar impetus.
In-Depth 2022-235
