Authors
The Food and Drug Administration (FDA) has issued new final rules aimed at modernizing mammography regulations. These rules, which were first proposed in March 2019, seek to improve the quality and accuracy of mammograms and ensure that patients have access to high-quality mammography services. The final rules have several significant revisions from the proposed rule, including changes in terminology and clarifications on various requirements. The new rules take effect September 10, 2024.
As described more fully below, FDA’s final rule, when effective, will require screening mammography providers nationwide to notify patients – in reports written in “lay language” – whether they have dense breasts and to encourage patients to discuss their risk of cancer with their health care provider.
One significant change is the substitution of the term “provider” or “healthcare provider” in place of references to “referring physician” in several paragraphs. This change reflects the reality that mammography is often ordered by a variety of health care professionals and not just physicians.
Another significant revision is the requirement that no accrediting body shall accept an application for accreditation from a facility that has had three consecutive failures to become accredited until one year after the most recent accreditation failure. This change is intended to ensure that facilities are held accountable for providing high-quality mammography services.
Additionally, facilities must now retain personnel qualification records of former employees for at least 24 months. This change is aimed at improving accountability and ensuring that personnel who are no longer employed at a facility are not able to perform mammography services elsewhere without proper qualifications. Similar to the treatment of medical records, a facility that closes or ceases to provide mammography services must make personnel records available by transferring the records to another facility or to the personnel.
The proposed term “digital accessory components” has been removed, and premarket requirements for devices used in mammography have been clarified, including a requirement that all devices used in mammography meet the applicable FDA premarket authorization requirements. This change is aimed at ensuring that devices used in mammography meet high-quality standards and that patients receive accurate and reliable results.
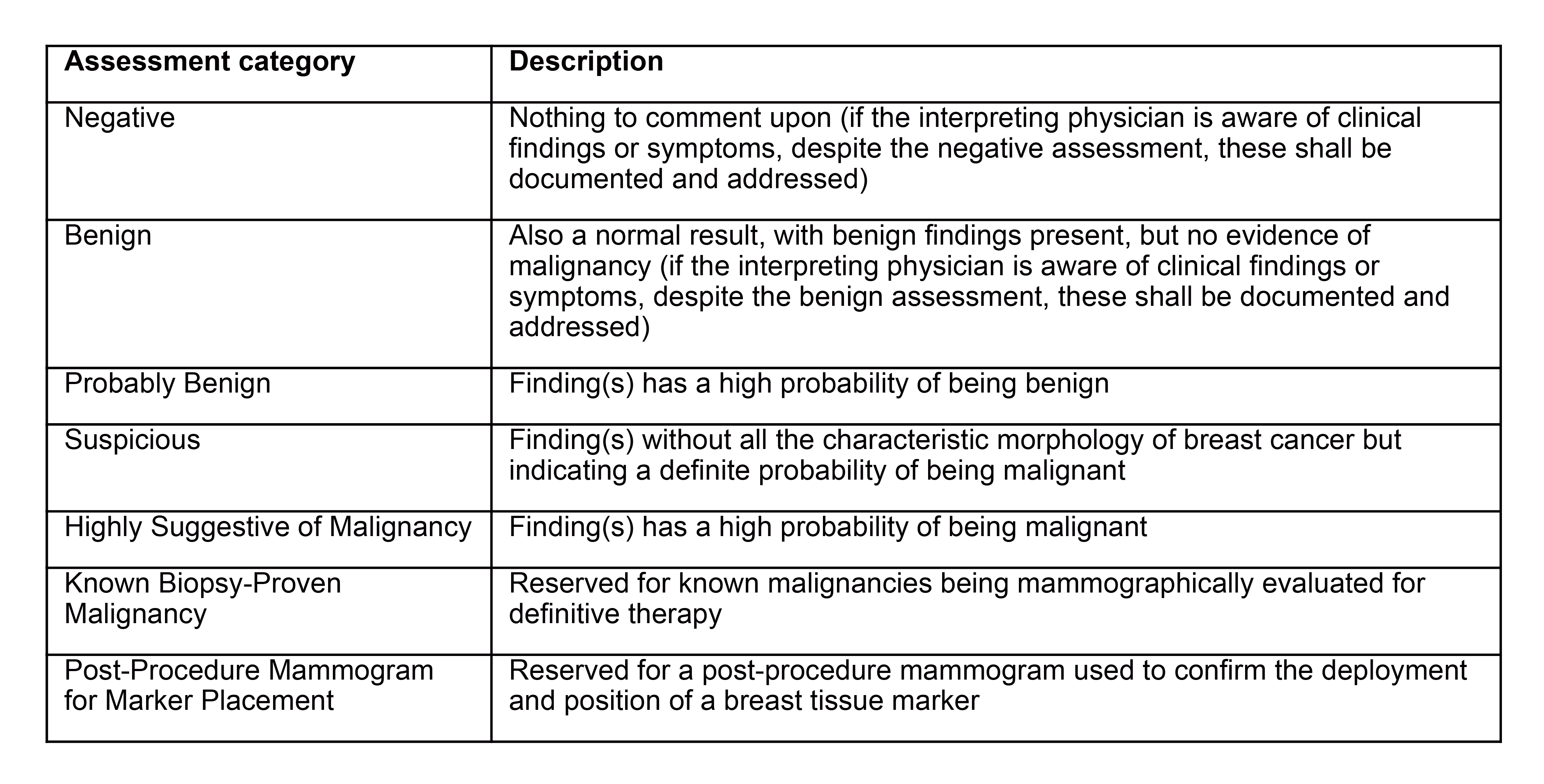
The FDA clarified the assessment statements that are required to be included in the written report of the results. This change is intended to ensure that patients receive clear and accurate information about their mammography results. The mammography report will be required to include the following information:
1. The name of the patient and an additional patient identifier;
2. The date of examination, facility name, and location (at a minimum, the location must include the city, state, ZIP code, and telephone number of the facility);
3. The name of the interpreting physician who interpreted the mammogram;
4. Overall final assessment of findings, classified in one of the following categories:
In cases where no final assessment category can be assigned because of an incomplete workup, one of the following classification statements must be assigned as an assessment with the interpreting physician stating the reasons why no final assessment can be made:
- “Incomplete: Need additional imaging evaluation” – This statement is to be reserved for examinations where additional imaging needs to be performed before an assessment category can be given; or
- “Incomplete: Need prior mammograms for comparison” – This statement is to be reserved for examinations where comparison with prior mammograms should be performed before an assessment category can be given.
If no assessment can be made because prior mammograms are needed for comparison, then a follow-up report with an assessment category must be issued within 30 calendar days of the initial report whether or not comparison views can be obtained.
5. Overall assessment of breast density, classified in one of the following categories:
a. The breasts are almost entirely fatty;
b. There are scattered areas of fibroglandular density;
c. The breasts are heterogeneously dense, which may obscure small masses; or
d. The breasts are extremely dense, which lowers the sensitivity of mammography.
6. Recommendations made by the interpreting physician to the health care provider about what additional actions, if any, should be taken. Note that all clinical questions raised by the referring health care provider must be addressed in the report to the extent possible, even if the assessment is negative or benign.
Other revisions to the rules include changes to the requirements for documenting and addressing clinical findings or symptoms in a patient whose mammogram assessment is negative or benign, clarifications on the deadline for sending mammography reports to self-referred patients, and requirements for maintaining a system for referring self-referred patients to a health care provider.
Mammography facilities will be required to provide each patient a summary of the mammography report written in lay terms within 30 calendar days of the mammographic examination. If the assessment of the mammography report is “Suspicious” or “Highly Suggestive of Malignancy,” the facility will be required to provide the patient with a summary of the mammography report written in lay language within seven calendar days of the final interpretation. Facilities also will be required to maintain a system for referring a patient who does not have a health care provider to a health care provider when clinically indicated.
Breast density notification language has also been revised to include additional information for patients with non-dense and dense tissue.
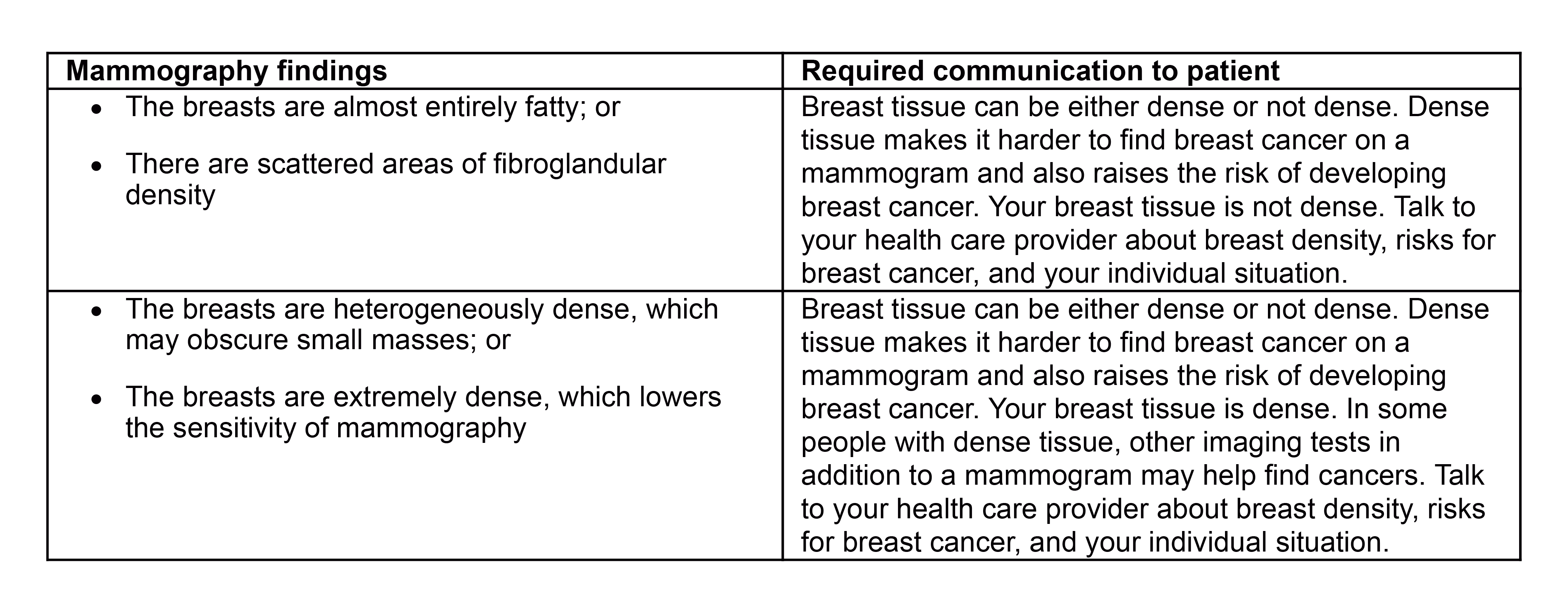
In the preamble to the final rule, the FDA acknowledges that 38 states have passed laws mandating notification of breast density. The new rules adopted by the FDA will set forth the minimum requirements for notification while preserving any state law that is more stringent than the FDA rules. In the instance that state law requires a specific statement addressing breast density, the FDA rules will likely preempt state law; however, mammography facilities should carefully analyze state law against the new FDA rules in order to comply with all applicable requirements.
Facilities are now required to maintain original mammograms and mammography reports in a permanent medical record of the patient for the longer of (1) a period of not less than five years, (2) a period of not less than 10 years if no additional mammograms of the patient are performed at the facility, or (3) a period, if any, mandated by state or local law. A facility may choose to retain original mammograms and mammography reports for a longer period of time as a risk management tool.
The new rules require mammography facilities to make the analysis of outcome data available to interpreting physicians. The outcome data must include, at a minimum, the following:
- Positive predictive value – percent of patients with positive mammograms who are diagnosed with breast cancer within one year of the date of the mammographic examination.
- Cancer detection rate – of the patients initially examined with screening mammograms who receive an assessment of “Incomplete: Need additional imaging evaluation,” “Suspicious,” or “Highly Suggestive of Malignancy” on the screening mammogram or on a subsequent diagnostic mammogram, the number of patients who are diagnosed with breast cancer within one year of the date of the initial screening mammogram, expressed arithmetically as a ratio per 1,000 patients.
- Recall rate – percentage of screening mammograms given an assessment of “Incomplete: Need additional imaging evaluation.”
Finally, the term “patient” has been substituted in place of references to “women” or “woman,” and the word “audit” has been added to clarify that the use of certain terms applies to the medical outcomes audit.
To sum up, the FDA’s new final rules modernizing mammography regulations are intended to improve the quality and accuracy of mammograms and to facilitate patients’ access to high-quality mammography services. The changes in terminology and requirements are designed to increase accountability and ensure that patients receive accurate and reliable results in a timely manner. The new rules will impose significant changes in how mammography facilities conduct breast cancer screening and detection.
Client Alert 2023-074